Answers section
Questions 2.16a 
- Why do you think that the invisible particles in one substance may not be identical to the particles in other substances? --------------------------------
- The pictures you have observed are of pure substances. Each of them consists of the same kind of simplest
invisible particles; so they cannot be divided into simpler substances by chemical means. Therefore they are called chemical elements.
- Define a chemical element. -----------------------------------------
- Name five examples of elements. --------------------------------------------
- Suppose one simplest particle of carbon combines chemically with one particle of oxygen, the product is
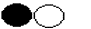 This is not a simple substance
because it can be chemically split into simpler substances
This is not a simple substance
because it can be chemically split into simpler substances  . It is called a compound.
. It is called a compound.
Define a compound. --------------------------------------------------------- - In the example given, a particle of carbon combines chemically with a particle of oxygen. They are the smallest particles that take part
in a chemical reaction, and are therefore called atoms. Here, the balls represent atoms.
What is an atom? ------------------------------------------------------------ - Some substances exist in small independent units, called molecules. That is, as small groups of chemically combined atoms but which exist
independently of one another. The combined atoms can be of the same element or of different elements. See the examples below.

Define a molecule. ------------------------------------------------------------------------- - Identify as free atoms, element, molecule, or compound what each of the following drawings represents. Some of them belong to more than one category.
In the diagram below, we have used the same colour to represent the same kind of invisible particles.


Answers to Questions 2.16a 
- Particles of the same substance, when crowded together, assume or give us a different colour from those of other substances. For example, sulphur particles appear yellow and copper is shiny red-brown. Also/Or, substances differ in many other ways such as hardness, softness, and boiling point. Some are safe and others are poisons; so their particles must be different in some ways.
- A chemical element is a pure substance that cannot be divided into simpler substances by chemical means.
- Oxygen, carbon, phosphorus, sulphur, copper, chlorine
- A compound is substance consisting of two or more elements chemically combined.
- An atom is the smallest particle of a substance that takes part in a chemical reaction.
- A molecule is the smallest particle that exists independently (or freely).
- Element
- compound, molecules
- element, molecules
- atoms, element, molecules
- (e) compound
NB: The atoms in (d) also qualify as molecules because they are free (independent) of one another.
