CHEMISTRY FORM 2
- 1.1 Structure of the atom
- 1.2 Atomic Number and Mass Number
- 1.3 Isotopes
- 1.4 Energy levels and electron arrangement
- 1.5 Development of the Periodic Table
- 1.6 Relative Atomic Mass and Isotopes
- 1.7 Ion Formation
- 1.8 Chemical Formulae
- 1.9 Chemical Equations

- 2.1 Alkali metals (Group I elements)
- 2.2 Alkali Earth Metals (Group II elements)
- 2.3 Halogens (Group VII elements)
- 2.4 Noble gases (Group VIII elements)
- 2.5 Properties and Trends Across the Periodic Table
- 3.1 Bond
- 3.2 Ionic bond
- 3.3 Giant ionic structure
- 3.4 Covalent bond
- 3.5 Co-ordinate bond
- 3.6 Molecular structures
- 3.7 Giant covalent structures
- 3.8 Metallic Bond
- 3.9 Types of bond across a period
- 3.10 Oxides of elements in Period 3
- 3.11 Chlorides of Period 3 elements

- 4.1 What is a salt?
- 4.2 Types of salt
- 4.3 Solubility of salts in water
- 4.4 Methods of preparing salts
- 4.4.1 Reacting a Metal with an Acid
- 4.4.2 Reacting an Acid with a Base (Neutralization)
- 4.4.3 Reacting an Acid with a Carbonate (or hydrogencarbonate of metal)
- 4.4.4 Combining elements Directly (Direct Combination of elements)
- 4.4.5 Precipitation (Double decomposition)
- 4.5 Action of heat on salts
- 4.6 Uses of salts
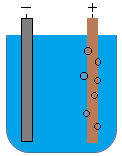
- 5.1 Electrical conduction
- 5.2 Electrical conductivity of molten substances
- 5.3 Electrical conductivity of substances in aqueous state
- 5.4 Electrolysis
- 5.5 Applications of electrolysis

- 6.1 Allotropes of carbon
- 6.2 Chemical properties of carbon
- 6.3 Carbon (IV) oxide
- 6.4 Carbon (II) oxide (CO)
- 6.5 Large scale production of sodium carbonate and sodium hydrogencarbonate
- 6.6 Effect of carbon (II) oxide and carbon (IV) oxide on the environment
- 6.7 Carbon cycle

Salts: Reacting an Acid with a Base (Neutralization)
4.0 Salts
4.4.2 Reacting an Acid with a Base (Neutralization)
Bases are metal oxides and hydroxides. Again the base and acid we select depends on the salt we want. This method is safe even for preparing salts of sodium, potassium, and calcium which react explosively with acids. Let us prepare sodium chloride, the table salt, as an example. Observe the video on preparation of sodium chloride salt.
(courtesy Youtube-Neutralisation of Sodium Hydroxide by Hydrochloric acid by Stuart Palmer)
Questions 4.4.2(a)
- Which acid is used to prepare sodium chloride and why?
- Name two bases that can be used to prepare sodium chloride.
- Is sodium chloride soluble or not? Apart from your experience at home, how do you know this?
- Describe how you would prepare sodium chloride from a suitable acid and base of sodium.
- Write a balanced equation for the reaction.
Answers to Questions 4.4.2(a)
The reaction between a base and acid produces salt and water only; so it is called neutralization. This method also works well with insoluble solid bases such as copper (II) oxide, zinc oxide, lead (II) oxide, iron (II) oxide, and lead (II) oxide. The same applies to their hydroxides.
Notice that although the less reactive metals such copper and silver do not react with dilute sulphuric or hydrochloric acids, their oxides and hydroxides do react with acids.
Observe the video showing how to prepare copper (II) nitrate or sulphate from copper (II) oxide below.
(courtesy Youtube-Making Salts - GCSE Science Required Practical by Malmesbury Education)
Questions 4.4.2(b)
- Describe the appearance of copper (II) oxide. Check on Chemistry Laboratory Chemicals (Module 1) if unsure.
- Describe how you would prepare copper (II) sulphate salt from copper (II) oxide and dilute sulphuric acid.
- Write an equation for the reaction (Copper (II) oxide is an insoluble solid).
- Why is the use of an indicator unnecessary in this case?
Answers to Questions 4.4.2(b)
Figure 4.4.2 shows copper (II) sulphate salt.

Figure 4.4.2 Copper (II) sulphate crystals
Neutralization method is suitable for all soluble salts.
