CHEMISTRY FORM 1




- 1.1 What is matter?
- 1.2 What is Chemistry?
- 1.3 What does matter consist of?
- 1.4 Are the particles in matter stationary?
- 1.5 Arrangement, distance, and attraction between particles
- 1.6 Properties of matter (volume, shape and compression)
- 1.7 Conductors and non-conductors
- 1.8 Sources of heat
- 1.9 Bunsen burner
- 1.10 Role of Chemistry in society
- 2.1 Pure substances
- 2.2 Mixtures
- 2.3 Separation of Mixtures
- 2.4 Separation of solid-solid mixture
- 2.5 Separation of insoluble solid-liquid mixture
- 2.6 Separation of soluble solid-liquid mixture (solution)
- 2.7 Separation of immiscible liquid-liquid mixture
- 2.8 Separation of miscible liquid-liquid mixtures (solution)
- 2.9 Separation of a liquid-gas mixture
- 2.10 Selecting and using appropriate methods of separating mixtures
- 2.11 Kinetic theory of matter
- 2.12 Classification by physical states
- 2.13 Effect of heat on physical states
- 2.14 Effect of impurities on melting and boiling points
- 2.15 Permanent and non-permanent changes
- 2.16 Definitions, chemical symbols and equations

- 3.1 Simple acid-base indicators
- 3.2 Universal indicators and pH scale
- 3.3 Reactions of acids with metals
- 3.4 Reactions of acids with carbonates and hydrogen-carbonates
- 3.5 Reactions of acids with bases
- 3.6 Effects of acids on substances
- 3.7 Applications of acids and bases

- 4.1 Composition of Air
- 4.2 Fractional distillation of liquid air
- 4.3 Rusting
- 4.4 Oxygen
- 4.5 Burning of substances in air
- 4.6 Atmospheric pollution

- 5.1 Candle wax and water
- 5.2 Reactions of metals with liquid water
- 5.3 Reaction of metals with steam
- 5.4 Preparation of hydrogen gas

Simple Classification of Substances and Separation of Mixtures: Definitions, Chemical symbols and equations
2.0 Simple Classification of Substances and Separation of Mixtures 
2.16 Definitions, Chemical symbols and equations
In section 1.3, we learnt that matter consists of tiny invisible particles. Moreover, there are different substances or types (iron, sulphur, iodine, etc) and states (solid, liquid, and gas).
Do all matter consist of the same particles?
Study the pictures of some selected elements provided below.

Questions 2.16(a)
From your observations:
- Why do you think that the invisible particles in one substance may not be identical to the particles in other substances?
- The pictures you have observed are of pure substances. Each of them consists of the same kind of simplest invisible particles; so they cannot be divided into simpler substances by chemical means. Therefore they are called chemical elements.
- Define a chemical element.
- Name five examples of elements.
- Suppose one simplest particle of carbon combines chemically with one particle of oxygen, the product is
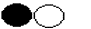 This is not a simple substance because it can be chemically split into simpler substances
This is not a simple substance because it can be chemically split into simpler substances  . It is called a compound. Define a compound.
. It is called a compound. Define a compound.
- In the example given, a particle of carbon combines chemically with a particle of oxygen. They are the smallest particles that take part
in a chemical reaction, and are therefore called atoms. Here, the balls represent atoms. What is an atom?
- Some substances exist in small independent units, called molecules. That is, as small groups of chemically combined atoms but which exist independently of one another. The combined atoms can be of the same element or of different elements. See the examples below.

Define a molecule. - Identify as free atoms, element, molecule, or compound what each of the following drawings represents. Some of them belong to more than one category.
In the diagram below, we have used the same colour to represent the same kind of invisible particles.


Answers to Questions 2.16(a)
Naming of substances
Naming of chemical substances follows a system.
- Names of compounds made up of two elements end in -ide.
- Where there is a metal, name of the metal comes first. For example, a compound of lithium and iodine is lithium iodide.
- Names of compounds having three or more elements, one of which is oxygen, end in -ite if it has fewer oxygen atoms than it can accommodate. It ends in -ate if it has the maximum possible number of oxygen atoms. For example, a compound of calcium, sulphur and oxygen is calcium sulphite if it has fewer oxygen atoms (CaSO3), or calcium sulphate if it has the maximum number of oxygen atoms (CaSO4).
Questions 2.16(b)
- Name the compounds A and B below, given that the brown ball represents copper, white represents oxygen, and black represents carbon.

- Name the compounds formed when the following elements combine chemically.
- sodium and oxygen
- magnesium and nitrogen
- potassium and chlorine
- zinc and bromine
- carbon and calcium
- calcium, oxygen and carbon
- oxygen, nitrogen and lead.
(Assume the maximum number of oxygen atoms in (f) and (g).)
Answers to Questions 2.16(b) 
Word equations for chemical reactions
Chemical changes (reactions) can be represented using word equations such as those given in Section 3.12. The substances that react with one another or undergo chemical change are called reactants.
Reactants are written on the left hand of the equation. The new substances formed are called products. Products are written on the right hand of the equation. In between, there is an arrow showing the direction of change.
The general equation is:

NB: The number of reactants and of products can be 1 or any other value depending on the case considered.
Questions 2.16(c)
Write the word equations for the reactions taking place between:
- Sodium and Oxygen
- Magnesium and Nitrogen
- Potassium and Chlorine
- Zinc and Bromine
- Calcium and Oxygen
Answers to Questions 2.16(c)
Chemical symbols
The following table shows the first 20 elements arranged in the order of their atomic numbers.

Other elements studied or mentioned in secondary school chemistry
| Chromium (Cr) | Manganese (Mn) | Cobalt (Co) | Copper (Cu) |
| Zinc (Zn) | Bromine (Br) | Mercury (Hg) | Silver (Ag) |
| Iron (Fe) | Lead (Pb) | Gold (Au) |
